Electrochemical Behavior of Copper Electrodes Modified with CS-GA-ZnO Np/Polysulfone Nanocomposites via Cyclic Voltammetry
Abstract
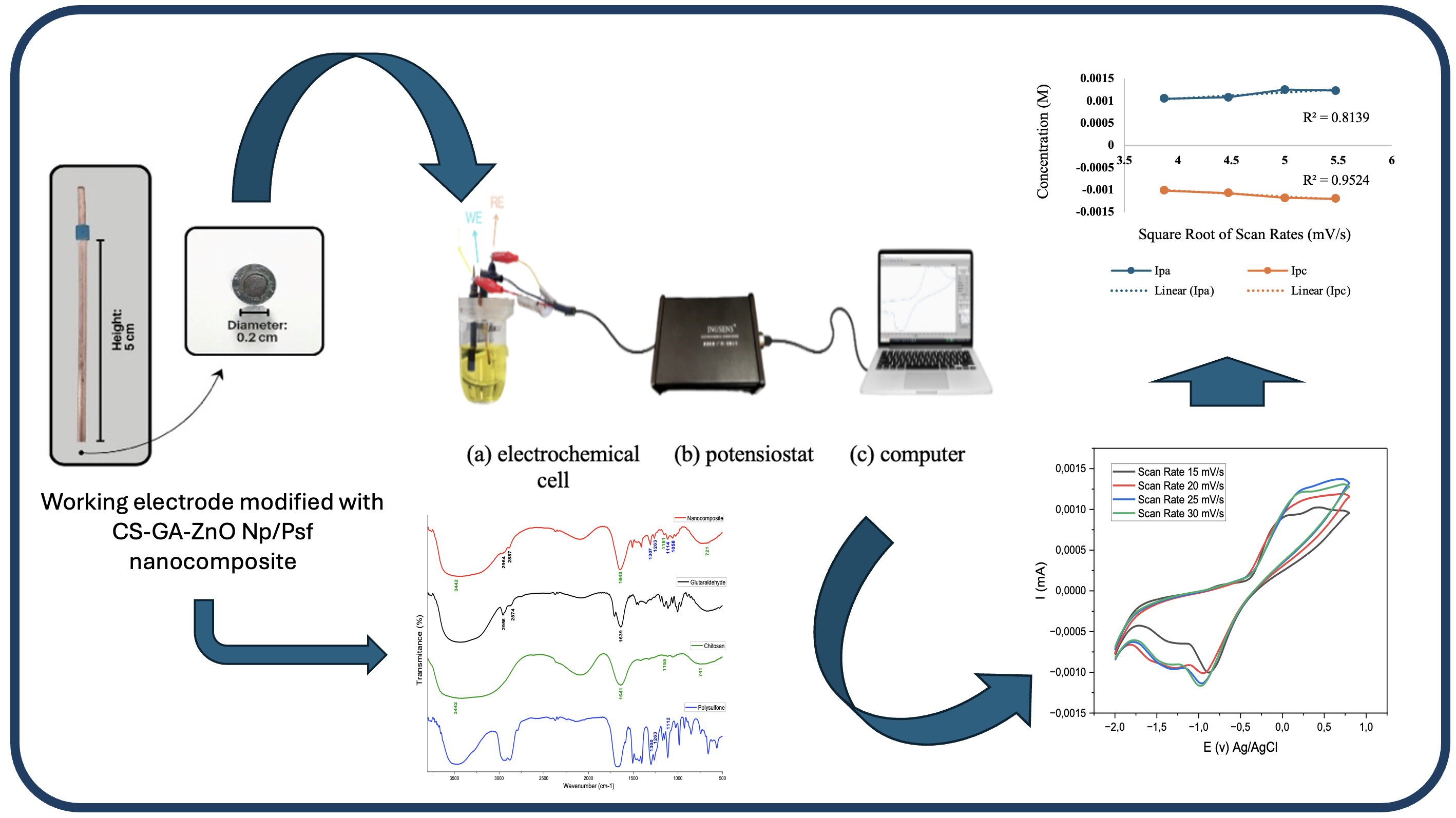
Copper (Cu) is an economical alternative electrode material with high electrical conductivity and electrochemical properties that support redox reactions. To enhance its electrochemical performance, the copper electrode was modified using a nanocomposite consisting of 5% chitosan (CS), 5% polysulfone (Psf), 0.9% Zinc Oxide nanoparticles (ZnO Np), and 25% glutaraldehyde (GA). This study aims to characterize and evaluate the electrochemical performance of the CS-GA-ZnO NP/Psf-modified copper electrode using cyclic voltammetry and FTIR spectroscopy. Cyclic voltammetry characterization was carried out using 0.03 M K₃[Fe(CN)₆] and 0.01 M analytical-grade glucose solutions, both prepared in 0.1 M KCl, with scan rate variations of 15–30 mV/s. The optimum scan rate for the K₃[Fe(CN)₆] solution was found to be 20 mV/s with an oxidation-to-reduction current ratio (Ipa/Ipc) of 1.01, while for glucose, the optimum scan rate was 30 mV/s with a ratio of 0.74. The FTIR spectrum showed a peak at 1643 cm⁻¹, indicating successful crosslinking between chitosan and glutaraldehyde through the formation of imine bonds (CH=N). These findings demonstrate that the CS-GA-ZnO NP/Psf nanocomposite effectively enhances electron transfer efficiency on the copper electrode surface. This modified electrode shows strong potential for application in electrochemical sensors, particularly for glucose detection.