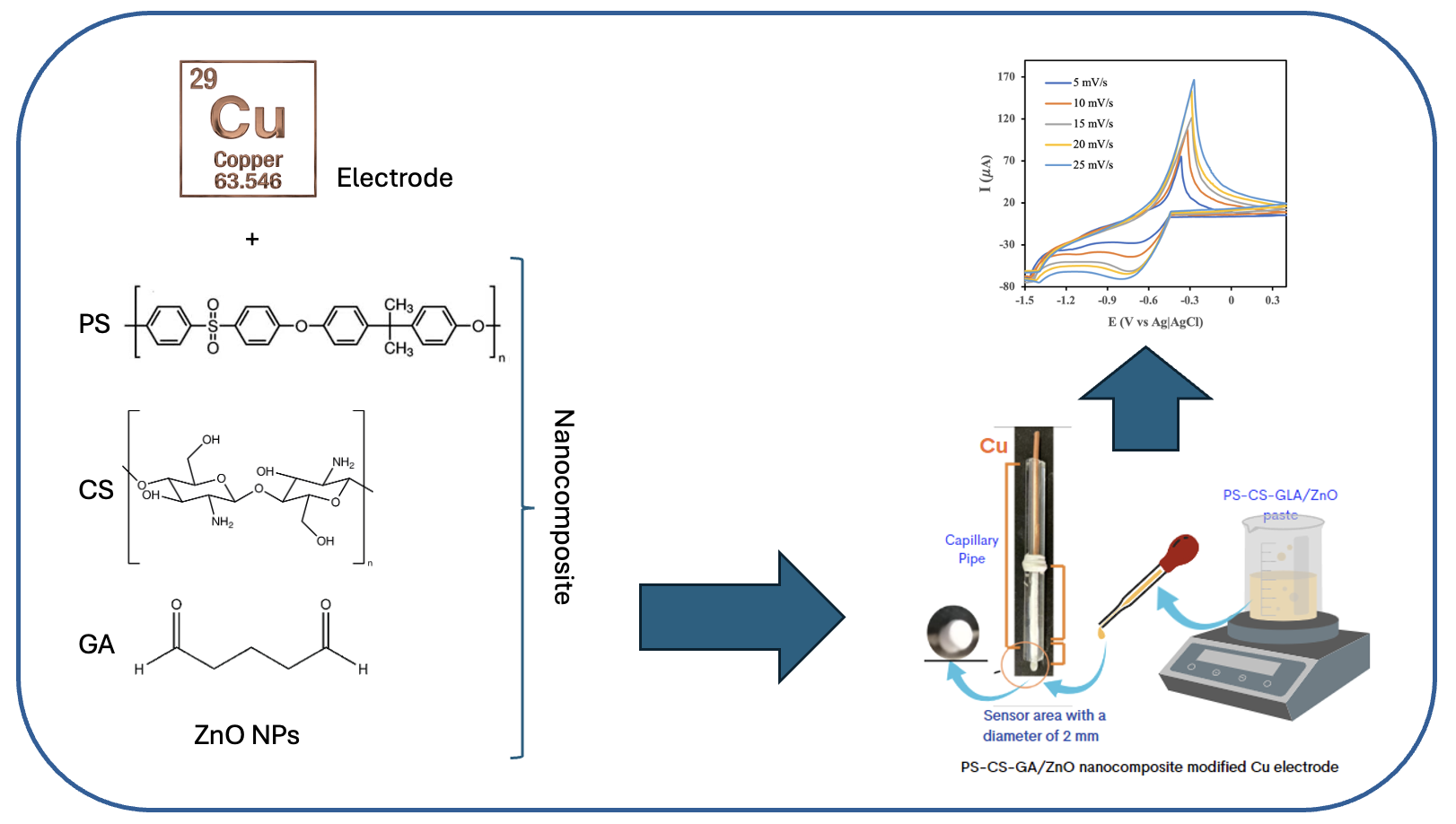
Evaluation of Scan Rate Influence on Cyclic Voltammograms of Copper Electrodes Coated with PS-CS-GA/ZnO Nanocomposite
Abstract
Copper (Cu) is widely utilized as an electrode material due to its high conductivity and relatively low cost. This study aims to develop nanocomposite-modified Cu electrodes using polysulfone (PS), chitosan (CS), glutaraldehyde (GA), and zinc oxide (ZnO) to form PS-CS-GA/ZnO-NP modified Cu electrodes. The nanocomposite was prepared in the proportions of 48.8% PS, 24.4% CS, 24.4% ZnO, and 2.4% GA using the phase inversion method. The characterization of the PS-CS-GA/ZnO nanocomposite-modified Cu electrode was conducted using cyclic voltammetry with a 0.01 M K3[Fe(CN)6] in 0.1 M KCl. The cyclic voltammogram profile was obtained at a scan rate of 25 mV/s, exhibiting an ipa/ipc ratio of 0.93 within a potential of -1.5 to +0.4 V vs Ag/AgCl over 15 cycles. The FTIR spectrum of the PS-CS-GA/ZnO nanocomposite thin film displayed a peak at 1668 cm-1, indicating the presence of C=N groups, which suggests a cross-linking interaction between CS and GA.