Evaluation of Ethyl Acetate Saponification Reaction: Comparison of the Accuracy of Conductometric and Titrimetric Methods
Abstract
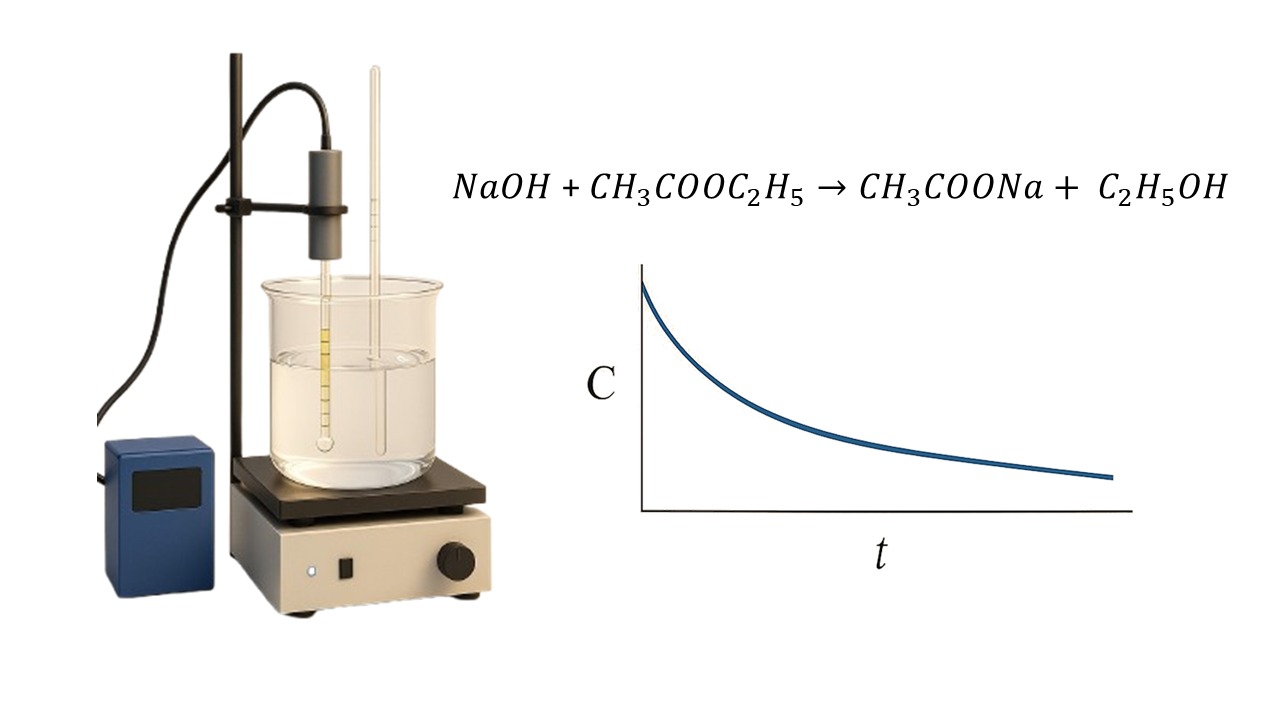
This study evaluated the kinetic characteristics of the ethyl acetate saponification reaction using two analytical approaches, namely conductometry and titrimetry, in a stirred batch reactor system at various temperature conditions. Both methods showed that the reaction followed an overall second-order pattern, in line with the theory of saponification reaction kinetics. However, significant differences were found in the conversion values and activation energy obtained. The titrimetric method showed higher reproducibility and accuracy, with an estimated activation energy of 37.40 kJ/mol. This study also confirmed that increasing the reactant concentration accelerated the reaction rate by increasing the frequency of effective intermolecular collisions. Overall, the titrimetric method is recommended as a more reliable approach in the study of saponification kinetics in this experimental system.