Room Temperature Synthesis of Aluminium(III)-Benzenedicarboxylate Complex from Two Different Al(III) Salts
Abstract
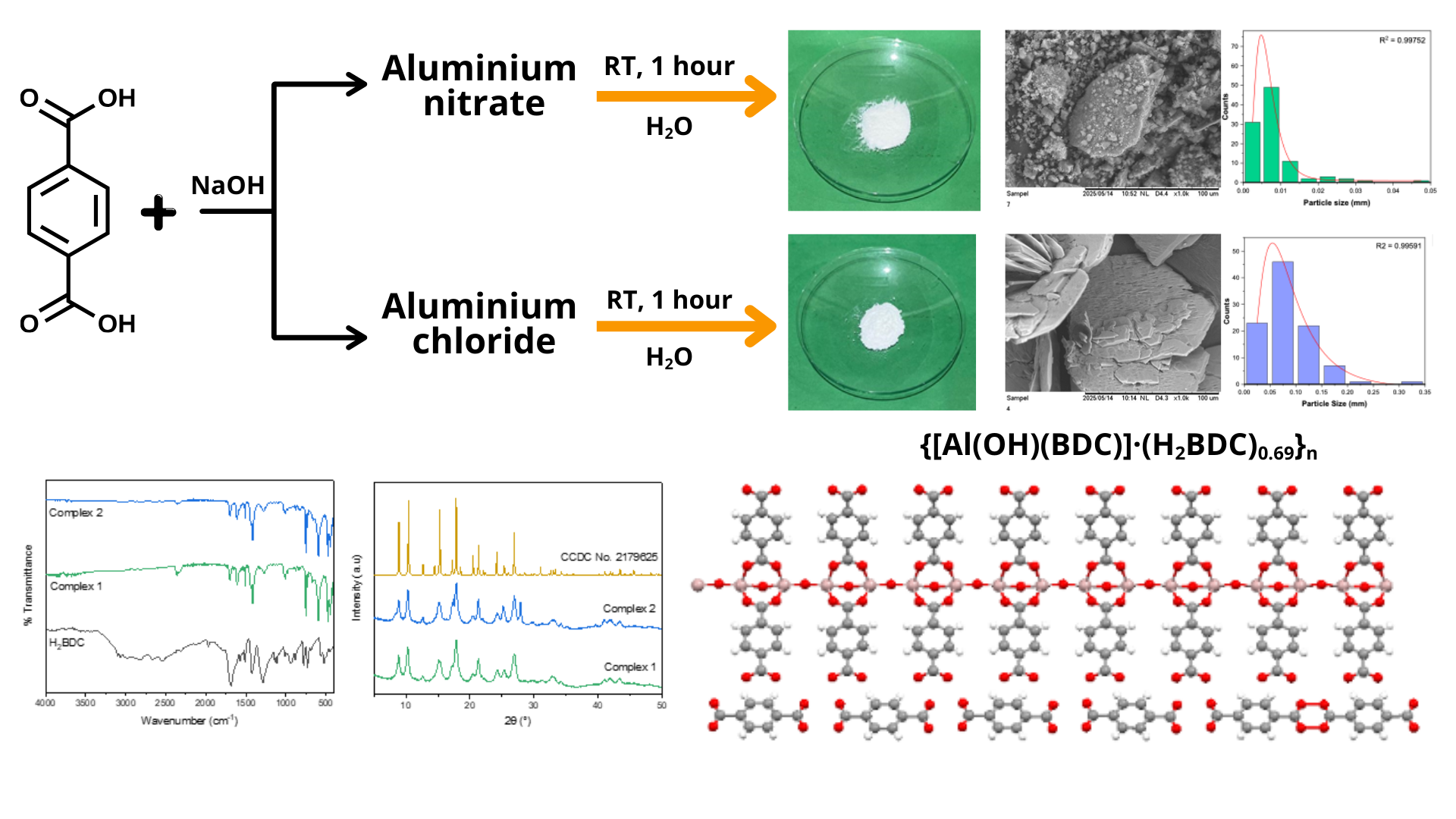
Al(III) complex with 1,4-benzenedicarboxylate ligand (Al-BDC) is a porous material that has the potential to be developed as an adsorbent or photocatalyst. This complex is often obtained by the solvothermal method at high temperature. This paper reports the synthesis of Al-BDC complex by solution method at room temperature from two different types of Al(III) salts, namely nitrate salt (complex 1) and chloride salt (complex 2). The synthesis of Al-BDC was conducted with Al(III):H2-BDC mol ratio of 2:3. The synthesized complexes were characterized by ATR-IR, powder XRD, UV Vis–DRS, and DTA–TGA. The results showed that white powders were obtained with a yield of 75.8% (complex 1) and 65.7% (complex 2). The presence of BDC ligands in both complexes was confirmed by the presence of typical absorption bands of C=O, C-O, and Al-O functional groups in their infrared spectra. Both complexes have different surface morphology and average crystallite sizes (28.63 nm – complex 1; 34.98 nm – complex 2), but the powder X-ray diffraction patterns, DTA-TGA thermograms, UV Vis-DRS spectra, and band gap energy values of both complexes are considerably identical. Powder XRD diffraction analysis of both complexes shows a pattern that is identical to the known compound with a formula of {[Al(OH)(BDC)]·(H2BDC)0.69}n(CCDC No. 2179625) in which the compound forms 3D polymeric structure with terephthalic acid occupies the voids.