Density Functional Theory Investigation of NO2 Gas Adsorption Properties on X12Y12 Nanocages (X= B, In and Y = As, P)
Abstract
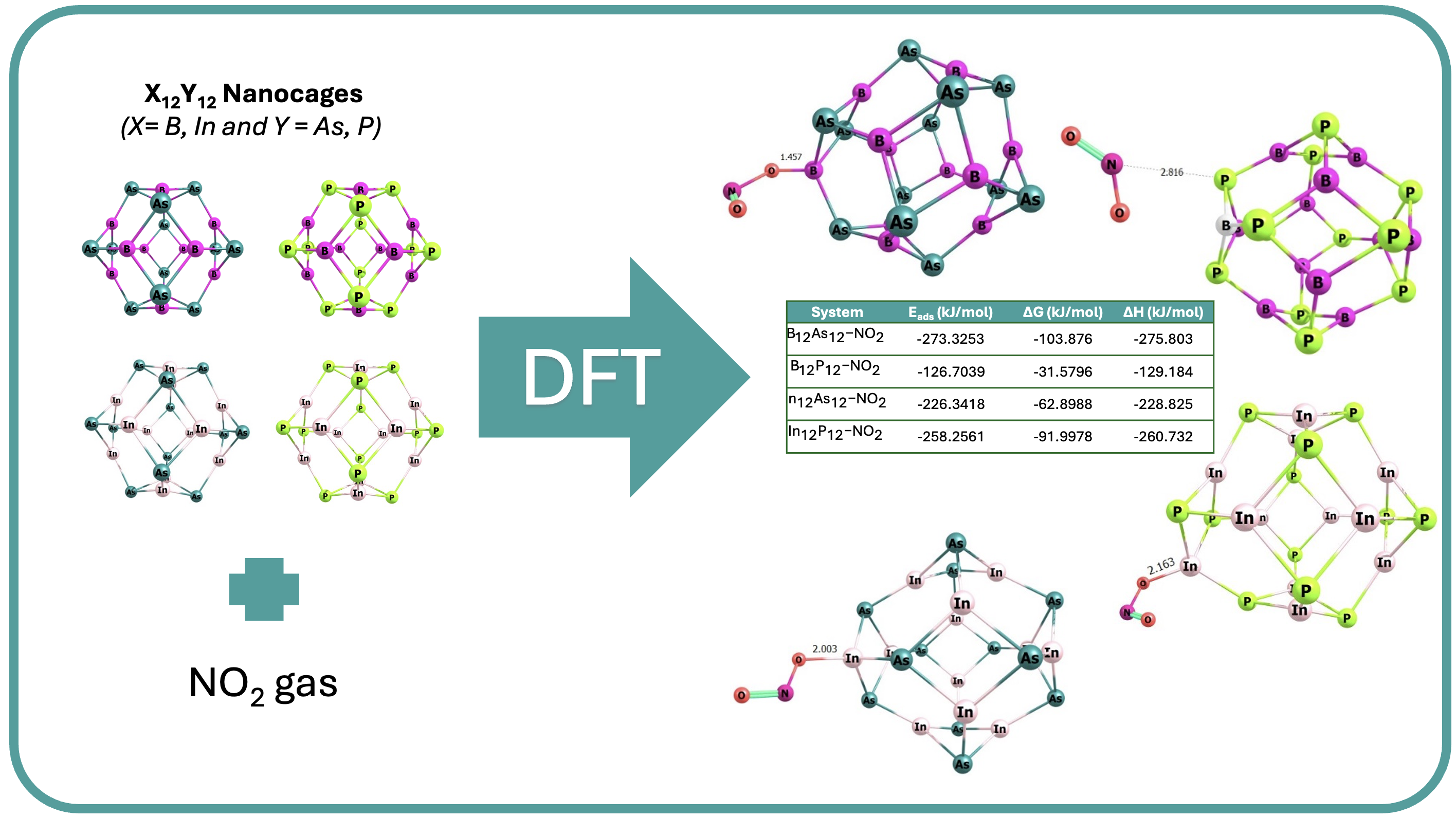
This study investigates the structural, electronic, and physical properties of X12Y12 (X = B, In, and Y = As, P) fullerene-like cages for NO2 adsorption. We employ Density Functional Theory (DFT) calculations with B3LYP methods, LANL2DZ basis set, and D4 dispersion correction to explore these properties, including ionization potential and electronic affinity linked to HOMO-LUMO gap energy. Moreover, this study explores global reactivity indices such as chemical potential, ionization potential, hardness, and softness. It also examines electronic properties, such as density of states (DOS), natural bond orbital (NBO), and electrostatic potential (ESP), along with chemical interactions like IGMH and AIMD, within the system. The findings demonstrate that the B12As12 nanocage exhibits high sensitivity to NO2 molecules, as evidenced by the bond interaction and adsorption energy of -273.33 kJ/mol, supported by UV-vis and IRI graphics, with the order of systems from the lowest to the highest adsorption energy toward NO2 : B12P12-NO2 < In12As12-NO2 < In12P12-NO2 < B12As12-NO2. AIMD simulations indicate that all nanocages can effectively adsorb NO2 within varying lengths by 1000 fs. This suggests the potential for high NO2 gas adsorption by the B12As12 nanocage. Further investigation is required to assess the potential of other nanocages.