Optimization of Methyl Orange Dye Photodegradation Using TiO2/Porous Ceramics with Response Surface Methodology
Abstract
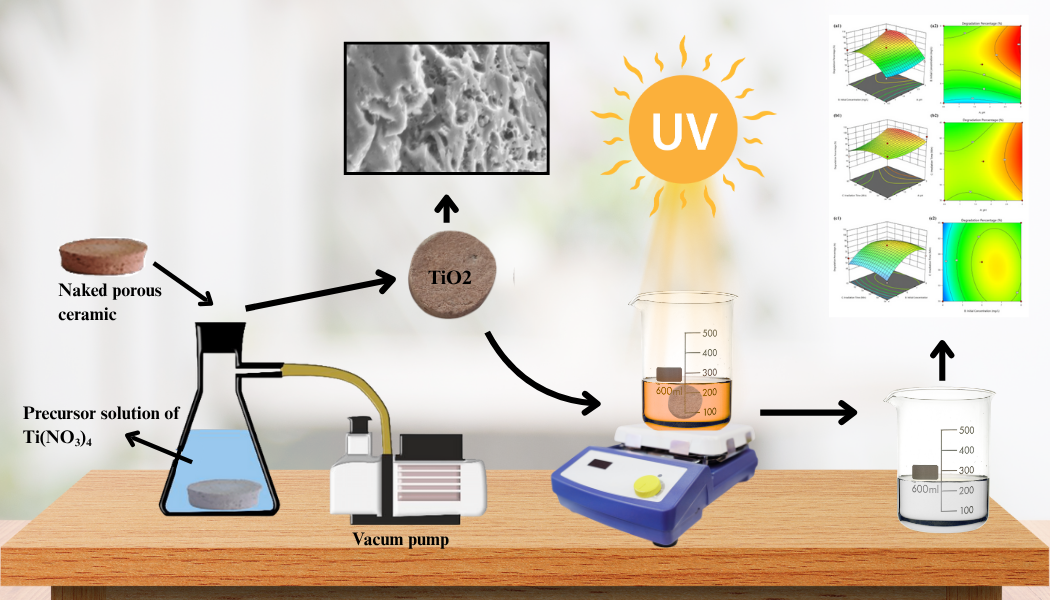
Environmental pollution from dye waste is one of the environmental problems that is of concern today. One method that is effective, reliable, and does not require a large cost is photodegradation. In this study, a TiO2/porous ceramic (TiO2/PC) photocatalyst was used which was synthesized by the sol-gel coating method. The resulting photocatalyst was then characterized using XRF, XRD, SEM, and SAA which were then applied to the photodegradation process of methyl orange (MO) dyestuffs. Optimization of photodegradation conditions of MO dyes using porous TiO2/PC catalysts was carried out using the Response Surface Methodology (RSM) method by Central Composite Design (CCD). The characterization results showed that the TiO2 phase was in the rutile phase with a level of 10.35%. The resulting photocatalyst has pores on the surface covered by TiO2 with a shrinking pore volume after impregnation from 0.076637 cm3/g to 0.064943 cm3/g with an average pore diameter from 4.51187 nm to 4.49999 nm. The optimal condition of degradation of MO dye occurred at pH 6, an initial concentration of 25 mg/L for 120 minutes, with a percentage of degradation of 89.54% and can be used up to four cycles. Thus, TiO2/PC photocatalysts have the potential to be used in the process of processing MO dye waste.