Thermodynamic and Infrared Spectroscopy Analysis of Tert-butyl Chloride and Hydroxide Nucleophilic Substitution Reaction Using Computational Method
Analisis Termodinamika Dan Spektroskopi Inframerah Reaksi Substitusi Nukleofilik Tersier Butil Klorida Dan Hidroksida Secara Komputasi
Abstract
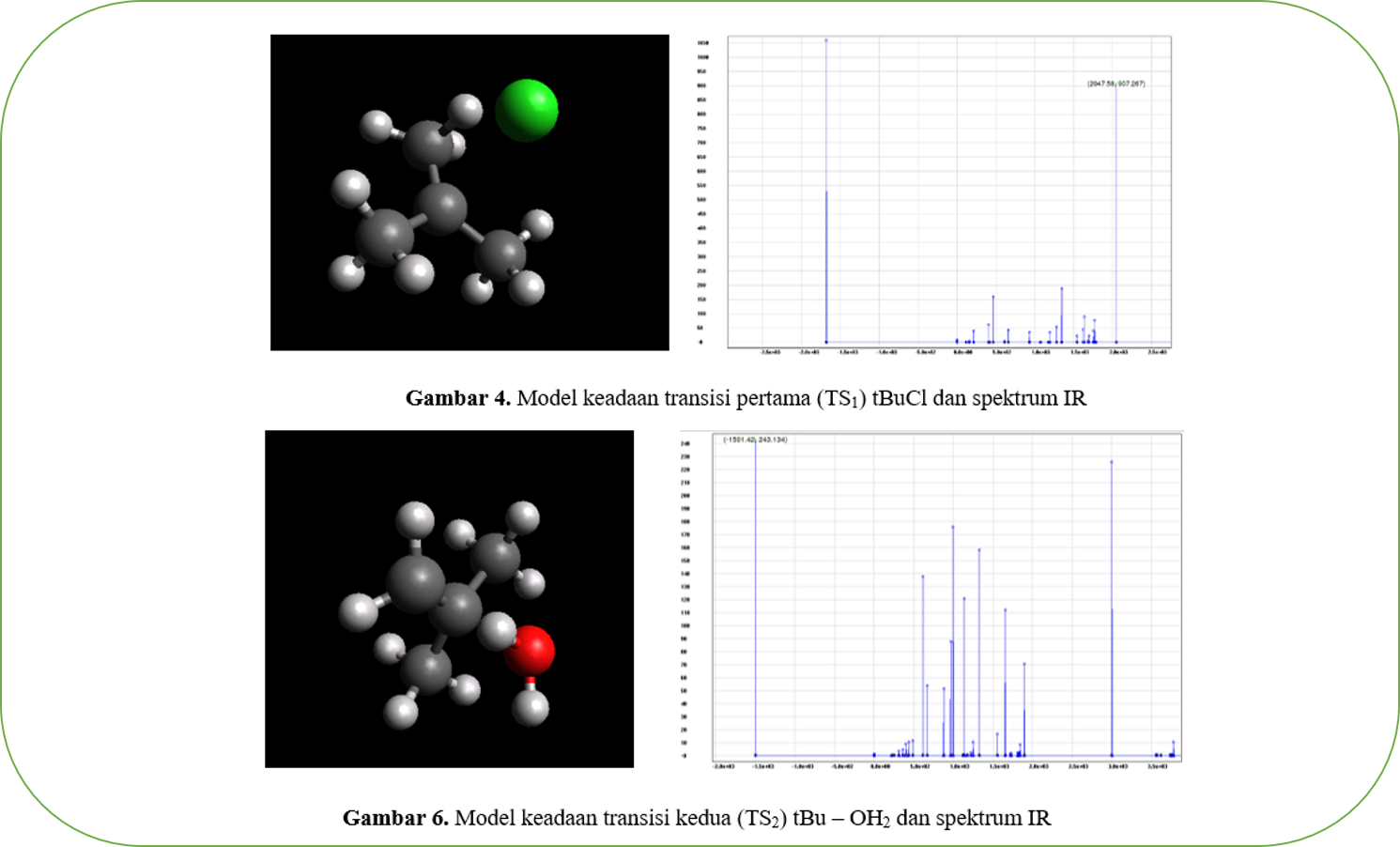
The nucleophilic substitution reaction is one of the important and commonly studied in organic chemistry reaction. The mechanism of how the reaction actually takes place and what species play a role in the system so that the product is formed is reviewed in this study. In this study, was modeled the nucleophilic substitution reaction of a tertiary compound butyl chloride by hydroxide ions. Computational modeling in this study, used software Putty, Marvin, NWChem, Avogadro, and ECCE Viewer. The final results of computational calculations obtained thermodynamic quantities in the form of activation energy 230.9478 kcal mol-1, reaction enthalpy -7101.74808 kcal mol-1, reaction entropy -40.178 cal/mol-K and Gibbs free energy of 4877.32262 kcal mol-1. Furthermore, the stability and reactivity of the molecules in this reaction were analyzed using molecular modeling and infrared spectroscopy.
Keywords: computational chemistry, nucleophilic substitution, tertiary butyl chloride