Synthesis and Characterization of Calcium Oxide Nanoparticles and Their Application in the Adsorption of Indigo Carmine
Abstract
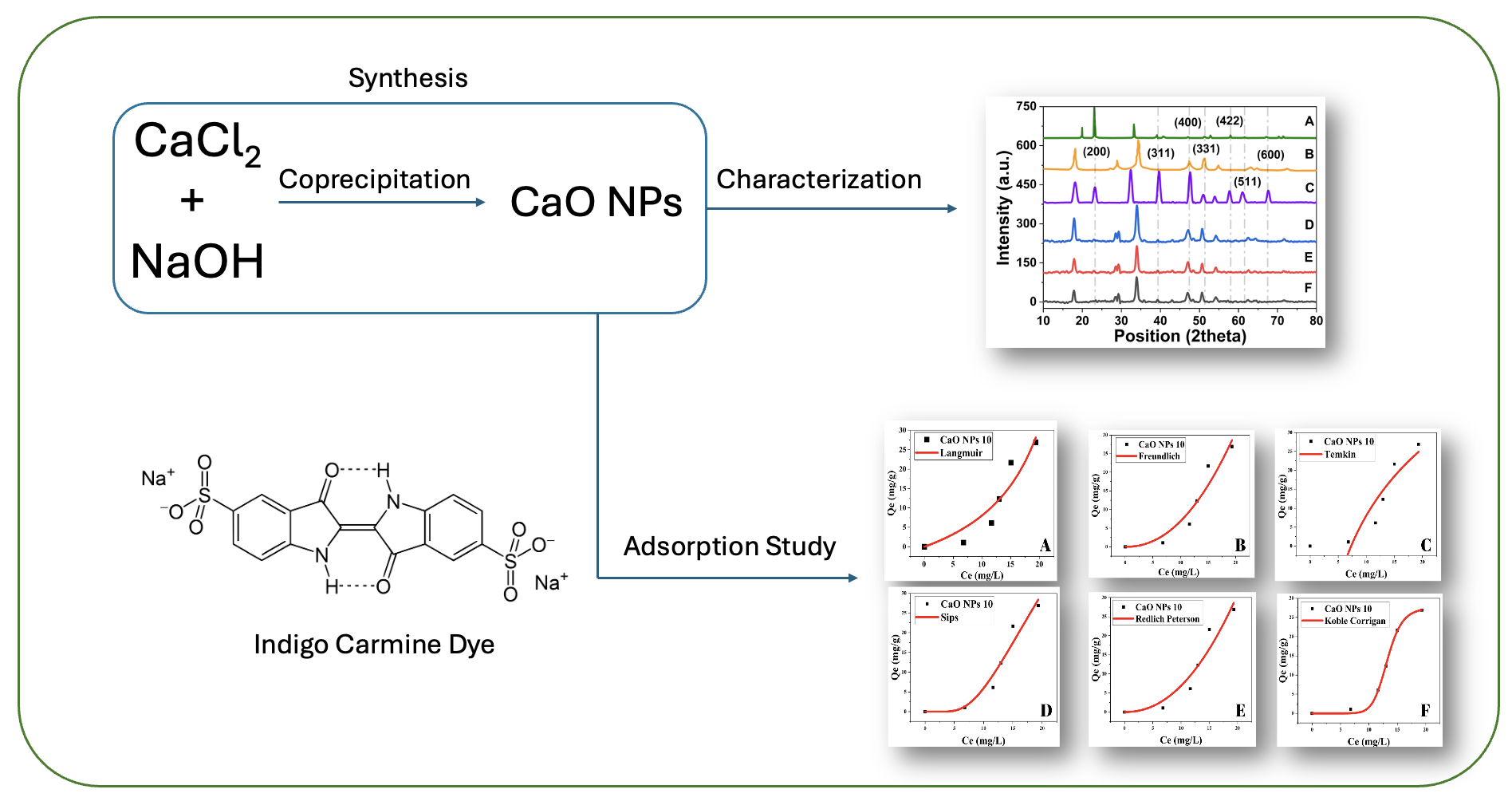
This research aims to determine the optimum conditions for the synthesis of CaO nanoparticles using the coprecipitation method, determine the characteristics of the CaO nanoparticles synthesized, and determine the effect of the performance of CaO nanoparticles as an adsorbent for indigo carmine dyes. CaO nanoparticles were successfully synthesized using the coprecipitation method at the optimum conditions of 1 M NaOH concentration and formation temperature of 400+200℃ with the highest % yield reaching 74.56%. The successful formation of CaO nanoparticles was proven by the appearance of a 2-theta diffraction peak of 23.12°; 39.2°; 57.9°; and 67.2° which is identical to the lattice structure of CaO with hkl indices (200), (311), (422) and (600) and a crystal size of up to 4.96 nm. SEM images support the formation of CaO nanoparticles with an average particle size of 98.1 nm and a varying size distribution. The IR spectrum of the formation of CaO nanoparticles with the appearance of Ca-O peaks at wavelengths of 3640, 1400, 860, and 791 cm-1. The adsorption capacity of indigo carmine on CaO nanoparticles was greatest at an adsorbent dose of 10 mg, adsorbate concentration of 100 ppm and a contact time of 50 minutes. The most appropriate adsorption isotherm model and adsorption kinetics model was the Koble-Corrigan model and the pseudo second order model, respectively.