The Moltens Salt Synthesis of Vanadium Doped Bi4Ti3O12 using Single Salt NaCl
Sintesis Bi4Ti3O12 Terdoping Vanadium dengan Metode Lelehan Garam Tunggal NaCl
Abstract
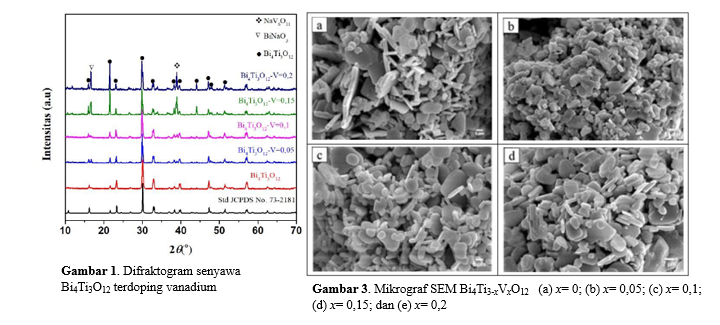
Bi4Ti3O12 is one of member threelayer Aurivillius compound family, which has the potential as a photocatalyst material with band gap energy 2.9 eV (427 nm). Therefore, to expand its utilization, the gap energy must be reduced. Doping with metal is one technique that can reduce the energy of a band gap. In this study, we synthesized vanadium doped Bi4Ti3O12 (Bi4Ti3-xVxO12 (x = 0, 0.05; 0.1; 0.15 and 0.2) by molten salt synthesis. Difractogram data shows that the Bi4Ti3O12 phase is formed with BiNaO3 and NaV6O11 impurities. The image of SEM showed that the particle shape of Bi4Ti3O12 is plate like. UV-Vis DRS spectra showed that the vanadium doped Bi4Ti3O12 have lower band gap energy than Bi4Ti3O12.
Keywords: Bi4Ti3O12, dopant vanadium, band gap energy
References
Wang W, Tadé MO, Shao Z. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem Soc Rev. 2015. 44(15):5371–408. http://dx.doi.org/10.1039/c5cs00113g
Liu Y, Zhu G, Gao J, Hojamberdiev M, Zhu R, Wei X, et al. Enhanced photocatalytic activity of Bi4Ti3O12 nanosheets by Fe3+-doping and the addition of Au nanoparticles: Photodegradation of Phenol and bisphenol A. Appl Catal B Environ. 2017. 200:72–82. http://dx.doi.org/10.1016/j.apcatb.2016.06.069
Xia A, Tan G, Ren H. Effect of Fe substitution on microstructure and properties of bismuth titanate thin films. Ceram Int. 2016. 42(1):1267–71. http://dx.doi.org/10.1016/j.ceramint.2015.09.061
Chen Z, Jiang X, Zhu C, Shi C. Chromium-modified Bi 4 Ti 3 O 12 photocatalyst: Application for hydrogen evolution and pollutant degradation. Appl Catal B Environ. 2016. 199:241–51. http://dx.doi.org/10.1016/j.apcatb.2016.06.036
Gu D, Qin Y, Wen Y, Li T, Qin L, Seo HJ. Electronic structure and optical properties of V-doped Bi4Ti3O12 nanoparticles. J Alloys Compd. 2017. 695:2224–31. http://dx.doi.org/10.1016/j.jallcom.2016.11.071
He R, Xu D, Cheng B, Yu J, Ho W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horizons. 2018. 3(5):464–504. http://dx.doi.org/10.1039/c8nh00062j
Xue P, Wu H, Lu Y, Zhu X. Recent progress in molten salt synthesis of low-dimensional perovskite oxide nanostructures, structural characterization, properties, and functional applications: A review. J Mater Sci Technol. 2018. 34(6):914–30. http://dx.doi.org/10.1016/j.jmst.2017.10.005
Zhao W, Jia Z, Lei E, Wang L, Li Z, Dai Y. Photocatalytic degradation efficacy of Bi4Ti3O12 micro-scale platelets over methylene blue under visible light. J Phys Chem Solids. 2013. 74(11):1604–7. http://dx.doi.org/10.1016/j.jpcs.2013.06.003
Januari T, Aini N, Barroroh H, Prasetyo A. The effect of synthesis time to particle size of Bi4Ti3O12 which synthesized using molten single salt NaCl method. IOP Conf Ser Earth Environ Sci. 2020. 456:12013. http://dx.doi.org/10.1088/1755-1315/456/1/012013
Handayani R, Safitri WN, Aini N, Hardian A, Prasetyo A. Synthesis and characterization of vanadium doped Bi4Ti3O12 as photocatalyst material. IOP Conf Ser Mater Sci Eng. 2019. 578:12017. http://dx.doi.org/10.1088/1757-899x/578/1/012017
Takle SP, Naik SD, Khore SK, Ohwal SA, Bhujbal NM, Landge SL, et al. Photodegradation of spent wash, a sugar industry waste, using vanadium-doped TiO2 nanoparticles. RSC Adv. 2018. 8(36):20394–405. http://dx.doi.org/10.1039/c8ra02869a